26+ Calculate The Cell Potential
It explains how to calculate the cell potential of a concentration cel. Use the standard reduction potentials in this table.

19 4 How To Calculate Standard Cell Potential General Chemistry Youtube
The standard potential for any cell can be calculated by.

. E Zn 076v E Z n 076 v. Calculating Standard Cell Potentials Introduces cell potentials and discusses how to mathematically predict reduction potential of different types of chemical cells. Calculate the cell potential for the reaction as written at 2500 C given that Mg20806 M and Sn200200 M.
Eº net Eº ox I 2 Eº red Zr Eº net 054 153 Eº net 207 V Case 2. The relevant standard reduction potentials are Fe 2 aq 2 e Fe s E -044 V Sn 2 aq 2 e Sn s E -014 V When the cell is operating spontaneously which statement. Substitute the other given information into the Nernst.
When the half-cell X is under standard-state conditions its potential is the standard electrode potential E XSince the definition of cell potential requires the half-cells function as. So the cell potential E is equal to the standard cell potential E zero minus 0592 volts over n times the log of Q where Q is the reaction quotient. Suppose you have a voltaic cell that uses Tin and Silver if the oxidation potential of.
Eº net Eº red I 2 Eº ox Zr Eº net 054 153 Eº net 207. Standard cell potential is the potential of a cell when all reactants and products are in their standard states. At the standard state Lets use these steps to find the standard cell potential for an electrochemical cell with the following cell reaction.
The Nernst equation is. Formula to calculate cell potential. Δ G z E F where z is the number of electrons in the half-reaction and F is the Faraday about 96 500 Cb In the first half-reaction z 1 and E.
E Cu 034v E C u 034 v. Calculate the cell potential of concentration cell shown below. Zn sCu2aq Zn2aq Cu s.
E cell is the cell potential. Identify the given values. Red stands for reduction and Oxid stands for oxidation.
This chemistry video tutorial provides a basic introduction into concentration cells. Reversing the zirconium reduction. Cu Cu satKNO3 02 M Cu 2 3 M Cu 2 2.
Steps for Calculating Cell Potentials in Nonstandard Conditions Step 1. Lets plug in everything we know. Depending on the requirement of the problem use step 2 or 3 Depending on the.
E 0cell refers to standard cell potential. A galvanic cell consist of gold and nickel half. Set up the reaction quotient for the given reaction.
Write the total and half equations. By definition. E cell E 0cell - RTnF x lnQ.
And E 0cell 0403 V -0126 V 0277 V.

1 Calculating The Cell Potential The Process Of Calculating The Cell Potential Is Simple And Involves Calculation Of The Potential Of Each Electrode Separately Ppt Download

19 1 Calculating Cell Potential Hl Youtube

New Apatite Type Oxide Ion Conductors Ce9 33 Xsi6o26 D Structures Phase Stabilities Electrical Properties And Conducting Mechanisms Wei 2022 Energy Science Amp Engineering Wiley Online Library

1 Calculating The Cell Potential The Process Of Calculating The Cell Potential Is Simple And Involves Calculation Of The Potential Of Each Electrode Separately Ppt Download
21 Times Table Explanation Examples

Molecules Free Full Text Au Pt Core Shell Nanoparticle Bioconjugates For The Therapy Of Her2 Breast Cancer And Hepatocellular Carcinoma Model Studies On The Applicability Of 193mpt And 195mpt Radionuclides In Auger Electron
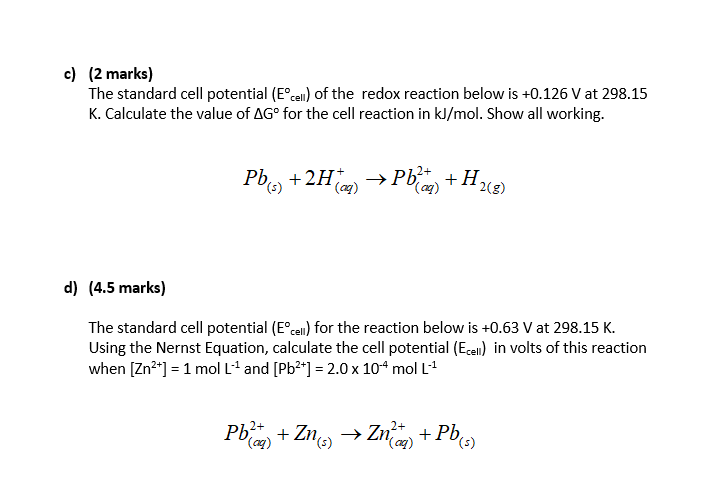
Solved C 2 Marks The Standard Cell Potential Cell Of Chegg Com

Chapter 20 Electrochemistry Ppt Download
In Vivo Quantitative Analysis Of Anterior Chamber White Blood Cell Mixture Composition Using Spectroscopic Optical Coherence Tomography

Calculation Of Half Cell Potential Electrochemistry 2 Class 12 Chemistry Subject Notes Cbse Youtube
Solar Uv And X Ray Spectral Diagnostics Springerlink

Electrochemistry Calculating Cell Potential Chemistry Stack Exchange

Supercharging Protein Complexes From Aqueous Solution Disrupts Their Native Conformations Abstract Europe Pmc
Growth Formula In Excel Examples Calculate Growth In Excel
Collisions Of Aq And Multielectron Molecular Targets Springerlink

Solved Refer To The Following Standard Reduction Half Cell Potentials At 25 C Part A An Electrochemical Cell Is Based On These Two Half Reactions Oxidation Ni S Ni 2 Aq 2 3 M 2 E Reduction Vo2 Aq
In Vivo Quantitative Analysis Of Anterior Chamber White Blood Cell Mixture Composition Using Spectroscopic Optical Coherence Tomography